Finally! Something new to talk about from my post-doc!
While it has been quite a struggle to juggle projects these past months, I have been able to submit and recently publish a study I have been intrigued about for several years already. Back in 2016 during a dissection of a tapir (see here), I noticed a peculiar morphological feature of the tapir shoulder muscles. Remarkably, it took six years to circle back to this osteological oddity thanks to an opportunistic dissection trip to the USA. The resultant study has now been published in The Anatomical Record, and you can read the paper here (or ask for it on ResearchGate).
Here’s how it all played out…
For over 50 years it has been known that the Malayan tapir (Tapirus indicus, the black-and-white one!) have muscles in their shoulder which are arranged slightly differently to other species of mammals – especially compared to their close living relatives, the horses and rhinoceroses. Not much is known about the musculature of other species of tapir, with only a few studies ever performed. And worse, the descriptions in those few studies do not line up with one another! So a tricky starting point! Back in 2020 I published a quantitative description of the forelimb muscles of the Malayan tapir (MacLaren & McHorse 2020). During that study my co-author Brianna McHorse and I confirmed the presence of a peculiar ridge on a feature of the scapula (the shoulder blade). The feature in question is called the scapula spine; this is a large, mostly flat piece of bone which arises perpendicular to the main “blade” of the shoulder-blade. On each side of the scapula spine there is a large muscle – one is called the supraspinatus (meaning “above the spine”), and the other is called the infraspinatus (meaning “below the spine”). These muscles attach to the scapula and connect it to the humerus (the upper arm bone) – both these muscles act as stabilising muscles in hooved mammals (such as tapirs, horses, cattle etc.). The peculiar thing about tapirs is the shape and location of their infraspinatus muscle, and the effect that has on the bony material of the scapula itself.
As mentioned above, ‘infra’ quite literally means ‘below’, and most self-respecting quadrupedal mammals have their infraspinatus muscle nestled in the section of their scapula which is below the scapula spine. This section is called the “infraspinous fossa” [a fossa, in this anatomical context, is a shallow depression in a bone – not to be confused with the Malagasy fossa, a carnivorous mammal made famous by the film Madagascar!]. The infraspinous fossa is normally the origin site for the infraspinatus muscle – and unsurprisingly, the supraspinous fossa is the origin site for the supraspinatus muscle. With me so far? Nothing weird yet, right? OK – enter: tapirs.

Tapir scapulae are universally weird. They have a substantial notch (a hollowed out section) just above the shoulder joint – sometimes the ligament which normally passes over this notch becomes completely ossified, forms a bony bridge, and then the tapir literally has a hole in its shoulder-blade! [see figure above] This phenomenon has not been documented in any other mammals other than great apes, and the shape of the tapir scapula is consequently quite unique among hooved mammals! And then there’s the supraspinous fossa. Tapir supraspinous fossae are very large relative to the infraspinous fossa, especially when compared to those of other living perissodactyls (horses and rhinoceroses) and most hooved mammals (including giraffes, antelope, pigs, etc.). Moreover, the supraspinous fossa of most tapirs sports a low ridge running from approximately the start of the scapula spine and ending at the dorsal margin of the scapula, roughly bisecting the supraspinous fossa. The ridge lies in the supraspinous fossa, so I used my imagination and decided to call it the “supraspinous ridge” [catchy, I know…]. THIS is the feature which stands out in my recent publication, for a couple of very good reasons.
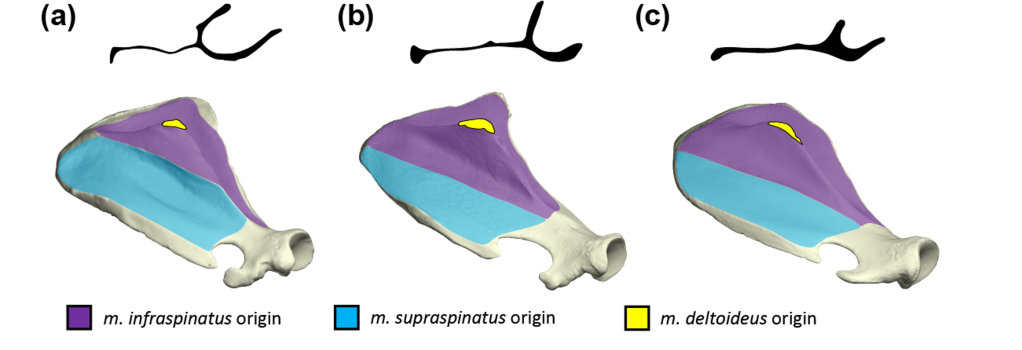
The supraspinous ridge has not been described before – it has been mentioned in passing, but not specifically described or compared across species. The purpose of my recent paper, therefore, was (1) to find out which tapirs showed a ridge, (2) what the ridge represented, and (3) whether there is any perceivable functional driver behind the presence of such a ridge. So let’s start at the beginning: who has a supraspinous ridge?
Within modern tapirs, I found that there was a clear supraspinous ridge in all species, but with a clear difference between the shape and placement of the ridge between the Malayan tapir and the three Neotropical species (lowland, Baird’s and mountain tapirs)*. The Malayan tapir supraspinous ridge pokes out from the scapula spine, whereas Neotropical species have the supraspinous ridge in the centre of their supraspinous fossae. Do already there is disparity in ridge expression. When I looked at extinct species, I found that all extinct New World tapirs exhibit the same ridge condition as modern New World tapirs – quite different to the Malayan tapir. When I looked further back in the fossil record, it seems that all tapir species which do not belong to the crown genus “Tapirus” do not show evidence of the supraspinous ridge. [There was tantalising evidence for the condition in the very early tapiroid ‘Hyrachyus’ (from the Messel pit in Germany), but it could not been confirmed in this study]. Since the scapula spine and ridge morphology only seems to be present in the genus Tapirus, and appears with two distinct and consistent morphologies, I think this could be used as a character for helping to work out family relationships between tapirs – a mission which is in urgent need of undertaking! It will be very interesting to see whether this trait is indeed useful for such a task, and I look forward to finding out in the future!

A species with † means that the species is extinct.
*small aside on the number of modern tapirs – there is some evidence for a fifth species (Tapirus kabomani), although the jury is still out somewhat on the validity of this species. The topic is covered nicely by Dr. Darren Naish in his TetZoo blog post, and I will leave the reader to read that post and decide their thoughts on the matter!
Ok, so I established which species exhibit the ridge, but what even IS the supraspinous ridge? Clues to the cause behind the ridge expression in tapirs could be found during comparative dissection, and as part of this study I was fortunate enough to take part in several! Comparative dissection of the shoulder region demonstrated that the infraspinatus muscle actually passes over the scapula spine (rather than originating only within the infraspinous fossa) and attaches on the supraspinous side of the scapula spine. In Malayan tapirs, the infraspinatus attaches on the scapula spine, whereas in the two Neotropical tapirs I studied (lowland tapir T. terrestris and mountain tapir T. pinchaque) the infraspinatus passes all the way over the scapula spine and attaches on almost 50% of the supraspinous fossa! The supraspinous ridge marks the border between the attachment sites of the infraspinatus and the supraspinatus muscles, otherwise known as the “epimysial border”. Thus, the supraspinous ridge of tapirs represents a very reliable osteological correlate for the location and presence of the supraspinatus and infraspinatus muscles. But what selective advantage would this change in muscle attachment actually present for Tapirus species?

In truth, this is where I am forced to speculate. The reality is that the shift in muscle attachments at the shoulder would not have conferred any great advantage from a biomechanical point of view, although it might be beneficial that the line of action of the infraspinatus muscle passes directly through the centre of the shoulder joint. At this point, I am truly baffled by the peculiar placement of the infraspinatus in tapirs. In my paper I speculate that this may be an adaptation for shoulder stability which is unlike that evolved in other hooved mammal species; the shoulder anatomy of the ancestor of Tapirus shifted, but that shift did not necessarily bring any specific advantageous (nor deleterious) effects on the locomotion of the group.
For now, I have chalked this one down as yet another enjoyable journey into the bizarre morphology of a wonderfully weird group of animals, and I hope to find out more about them during the remainder of my postdoctoral project!